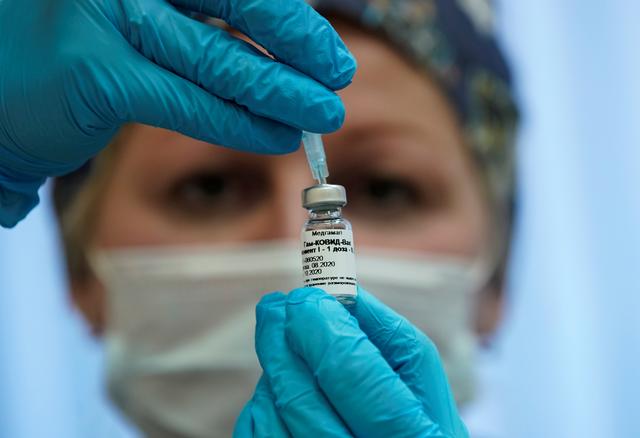
The US Food and Drug Administration granted emergency use authorization for a vaccine developed by Johnson & Johnson. The company will start shipping millions of doses early this week. As per the plan, J&J will provide the United States with 100 million doses by the end of June.
America has already been assured of getting 600 million doses of the Covid-19 Vaccines, made by Pfizer-BioNTech and Moderna. These vaccines are due to be delivered over the next four months. This will be enough to cover every American adult who wants to be vaccinated.
The new vaccine differs markedly from the two already in use in the United States. Here is how they compare.
Unlike the Pfizer-BioNTech and Moderna vaccines, the Johnson & Johnson vaccine will be administered in a single shot.
“Our goal all along has been to create a simple, effective solution for the largest number of people possible, and to have maximum impact to help end the pandemic,” Johnson & Johnson Chief Executive Officer Alex Gorsky said in a statement when his company’s results were released in late January.
The Johnson & Johnson vaccine uses a different method to prime the body to fight off COVID-19: a viral vector called Ad26. Viral vectors are common viruses that have been genetically altered so that they do not cause illness but can still cause the immune system to build up its defenses. The Pfizer-BioNTech and Moderna vaccines use messenger RNA to do that.
Pfizer: 95% effective at preventing symptomatic infections, nearly 100% effective at preventing severe infections (one case among more than 18,000 vaccinated individuals in the trials), after two doses.
Johnson & Johnson: 66% effective at preventing moderate to severe infections, 85% effective at preventing severe infections, after a single dose. (There were five confirmed severe cases among more than 19,000 fully vaccinated individuals in the trials, meaning people who’d received the shot at least 28 days prior.)
The Johnson & Johnson vaccine is rated as highly effective at preventing serious illness and death, as the Pfizer-BioNTech and Moderna vaccines are. It is also very effective at preventing milder illness, though a bit less so than those two.
The Johnson & Johnson vaccine can be refrigerated for three months, making transportation and storage far less of a challenge. Furthermore, it does not have to be stored at extremely low temperatures like the Pfizer-BioNTech vaccine.
The Johnson & Johnson vaccine is also less prone than the Pfizer-BioNTech and Moderna vaccines to trigger the kinds of side effects that require monitoring after the injection,