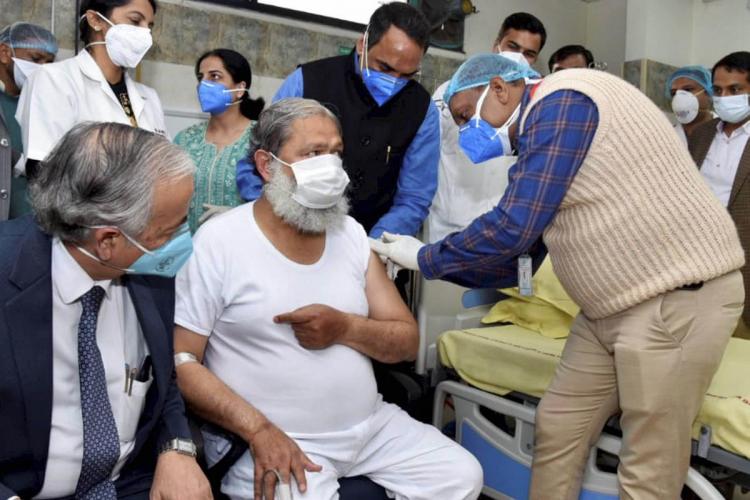
In December Haryana Health Minister Anil Vij, tested positive for Covid-19 days after receiving a dose of the Bharat Biotech’s COVID-19 vaccine. It was reported that he has been admitted to Gurugram’s Medanta Hospital in a critical condition.
According to IANS report, the Minister is suffering from lung infection, and is getting treatment under Dr Sushila Kataria, senior director of Internal Medicine Department at Medanta.
Vij was shifted to Medanta on Tuesday evening from the Haryana government’s Post-Graduate Institute of Medical Sciences (PGIMS) in Rohtak where he was admitted on Saturday and had received convalescent plasma therapy.
ALSO READ| Breaking: Days After Getting Trial Covaxin Shot, Haryana Minister Anil Vij Tests Corona Positive
Earlier, the institute had informed that the Minister had moderate Covid-19 with bilateral viral pneumonia which is why he was shifted to the private facility in Gurugram after his family found his health condition not improving there. Before admitting at PGIMS, Vij was treated at the Civil Hospital in Ambala.
Anil Vij Received Bharat Biotech’s COVID-19 Vaccine Last Month
In phase 3 trails of Covaxin the minister was offered to be a volunteer, Covaxin is the indigenous Covid-19 vaccine candidate developed by Hyderabad-based Bharat Biotech. He received a jab of the two-dose vaccine on November 20 at the Civil Hospital in Ambala Cantonment. But he tested positive for Covid-19 on December 5, which raised a concern regarding the vaccine’s efficacy among the public.
However, Bharat Biotech issued a statement saying that clinical trials of the vaccine are based on a two-dose schedule that is given in 28 days gap; its efficacy will be determined in two weeks after volunteers receive the second dose, according to the company’s statement.
The Union Health Ministry had also clarified that Covaxin is a two-dose vaccine and that the Haryana Health Minister was given only the first dose a fortnight before he tested COVID positive.
ALSO READ| Here Is An Explainer On The Possible Reason Behind Covaxin Volunteer Anil Vij Testing Covid-19 Positive
Covaxin Is Safe, Developed On Time-Proven Tech: Bharat Biotech
Bharat Biotech, which is conducting Phase-3 clinical trials of Covaxin in over 25 centres across India, applied for emergency use authorisation for its COVID-19 vaccine last week based on Phase 1 and 2 trial data.
After deliberating upon its applications, an expert committee of the Central Drugs Standard Control Organisation (CDSCO) asked the company to give an additional safety and efficacy data from the ongoing phase 3 clinical trial in the country for further consideration.
However, Krishna Illa, Bharat Biotech’s chairman and managing director, claimed that Covaxin is safe and built on a time-proven technology.
Meanwhile, the trials on animals (monkeys) have shown the efficacy and the Phase 1 and 2 human trial data has also proven it to be safe, he said while speaking virtually at a programme organised by the industry body FICCI.
Phase-2 trail was done on 2,000 people which is not a small number, Illa elaborated while adding that the vaccine can be given to any age group of people.
NITI Aayog member (health) Dr V K Paul on Tuesday informed media persons that the applications of Bharat Biotech, Serum Institute and Pfizer for emergency use authorisation for their COVID-19 vaccines are being examined by the subject expert committee on COVID-19 of CDSCO. He also assured that the drug regulator seeking more data from these companies will not impact the vaccine-roll out timeline.