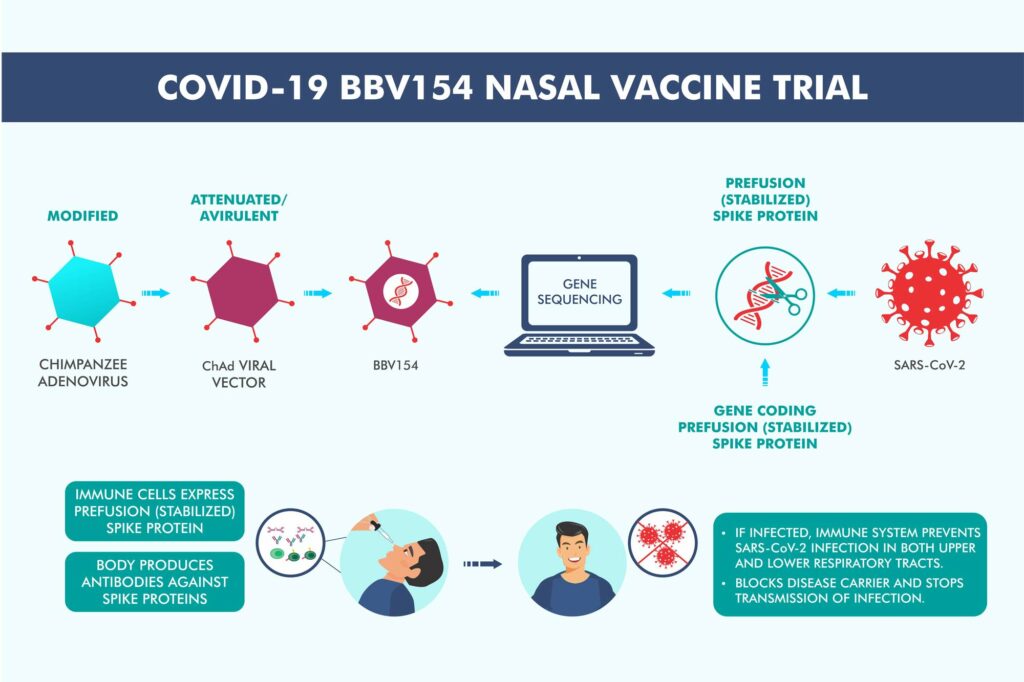
BBV154, an intranasal vaccine candidate, being developed by Bharat Biotech, is in the early clinical stage of development in India.
This has been announced by the Minister of State for Health Ashwini Choubey in the Rajya Sabha on Tuesday. Choubey was responding to a question on the status of launching a nasal vaccine for Covid-19.
“The interval period between the two doses is presently 21 days. The schedule will be finalized after the phase I clinical trial data. Currently, in phase I clinical trial, single dose and two dose schedules are being tested,” the minister said.
“Intranasal vaccines are administered as a nasal spray and offer a needle-free approach for vaccine administration”, Choubey said in a written reply.
BBV154
- An intranasal vaccine stimulates a broad immune response – neutralizing IgG, mucosal IgA, and T cell responses.
- Immune responses at the site of infection (in the nasal mucosa) – essential for blocking both infection and transmission of COVID-19.
- The nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa.
- Non-invasive, Needle-free.
- Ease of administration – does not require trained health care workers.
- Elimination of needle-associated risks (injuries and infections).
- High compliance (Ideally suits for children’s and adults).
- Scalable manufacturing – able to meet global demand.