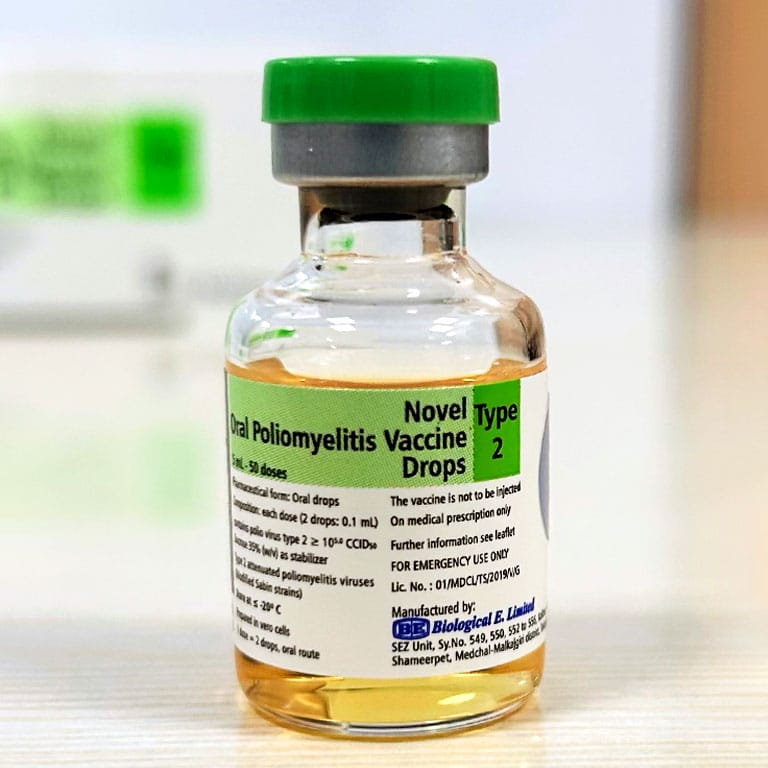
In a major step towards eradicating polio worldwide, Biological E. Limited (BE), a Hyderabad-based vaccine and pharmaceutical company, has announced.
The World Health Organization (WHO) has granted Pre-qualification (PQ) status to their Novel Oral Polio Vaccine type 2 (nOPV2). This is BE’s 10th pre-qualify vaccine. The new oral vaccine significantly reduces the risk of circulating vaccine-derived Poliovirus type 2 (cVDPV2) outbreaks. It aimsx at immunizing countries affected by cVDPV2 outbreaks, marking a crucial moment in the fight against polio.
The threat of circulating vaccine-derived Poliovirus type 2 (cVDPV2) outbreaks can be tackled with the use of nOPV2. This next-generation live, attenuated oral vaccine has improved genetic stability, which means it has a much lower chance of causing new outbreaks in low-immunity areas compared to its predecessor, the Sabin poliovirus type 2 (mOPV2) vaccine.
Extensive clinical trials have rigorously evaluated the safety and effectiveness of nOPV2, with promising results published in The Lancet (2019-2024). Moreover, real-world deployment in outbreak regions has shown that nOPV2 can significantly decrease the incidence of cVDPV2 outbreaks, protecting communities from it.
BE has become a key player in the production of the nOPV2 vaccine. They received a grant from the Bill and Melinda Gates Foundation (BMGF) to help meet the growing global demand. In collaboration with PT Bio Farma (PTB) in Indonesia.
The first manufacturer of the nOPV2 vaccine to receive WHO Pre-Qualification in January 2024, BE successfully receive technology from PTB and qualify large-scale manufacturing facilities that produce over 500 million doses of the nOPV2 vaccine annually.
BE approve by Indian regulatory authorities to manufacture the vaccine for export purposes. Ms. Mahima Datla, Managing Director of Biological E.
Limited, said: “We are pleased to be a part of the global effort to eradicate it. Our collective quest to eradicate polio marks a significant milestone with the WHO prequalification of nOPV2.
This vaccine has been specifically design to address concerns about Vaccine-Associated Paralytic Polio (VAPP).
Which has occurred in approximately 2 to 4 cases per million births with the traditional OPV due to the vaccine virus reverting to a virulent form.”
Read Also- India Reports a Rare Case of Vaccine-Derived Polio Virus Transmission from Child to Father Post
Ms. Datla further expressed BE’s gratitude for the collaboration with PT Bio Farma (PTB) and the support of a grant from the Gates Foundation: “Collaborating with PTB is a privilege and we extend our heartfelt gratitude to the Gates Foundation for entrusting us with the responsibility of manufacturing nOPV2.
Together we are committed to advancing the cause of global health equity and guaranteeing that no child is affect by the devastating effects of polio. The significance of this milestone extends beyond scientific achievement; it represents a beacon of hope for millions of children and families around the globe. The achievement of administering over 1 billion doses of nOPV2 in outbreak regions is crucial to realizing the dream of a polio-free world.’’