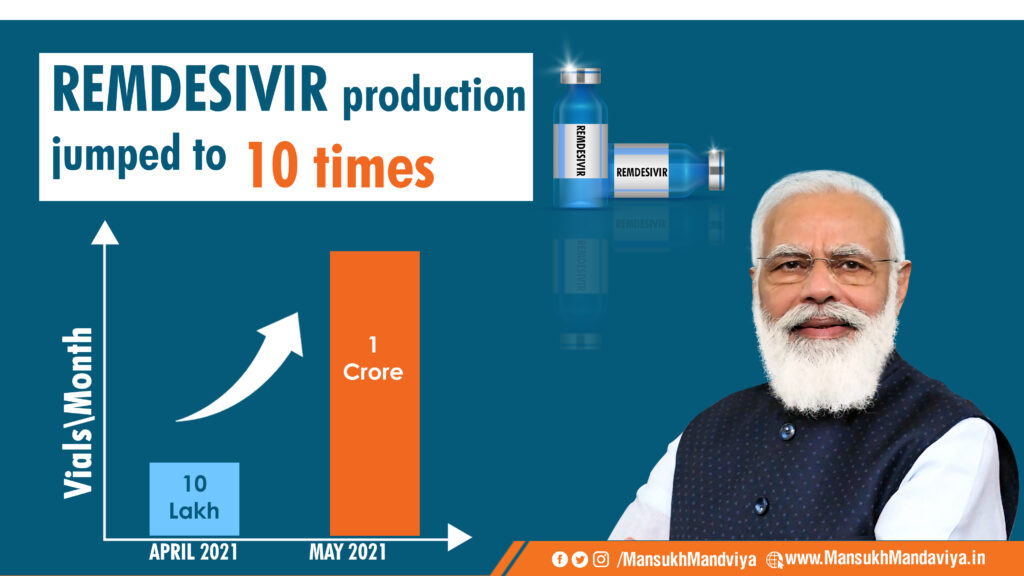
Minister of State for Chemicals and Fertilizers Shri Mansukh Mandaviya informed that the production of Remdesivir is ramped up ten times from 33,000 vials/day on 11th April 2021 to 3,50,000 vials/day today under the leadership of Prime Minister Shri Narendra Modi.
The Minister further informed that the Government has also increased the number of plants producing Remdesivir from 20 to 60 plants within a month. Now the country has enough Remdesivir as the supply is much more than the demand, he added.
Shri Mandaviya said that the Government has decided to discontinue the Central Allocation of Remdesivir to States. He has directed the National Pharmaceuticals Pricing Agency and CDSCO to continuously monitor the availability of Remdesivir in the country.
Government of India has also decided to procure 50 lakh vials of Remdesivir to maintain it as a strategic stock for the emergency requirement, he added.
I am delighted and satisfied to inform you all that the Production of Remdesivir is ramped up ten times from
just ~33,000 vials/day on 11th April 2021 to ~3,50,000 vials/day today under the astute leadership of Hon’ble PM Shri @narendramodi Ji. (1/3) pic.twitter.com/Vy2RzrsYMQ— Mansukh Mandaviya (@mansukhmandviya) May 29, 2021
The Union minister said that the Centre has decided to procure 50 lakh vials of Remdesivir to maintain it as a strategic stock.
“But I have also directed @nppa_india & @CDSCO_INDIA_INF to continuously monitor the availability of Remdesivir in the country.
Government of India has also decided to procure 50 lakh vials of Remdesivir to maintain it as a strategic stock for the emergency requirement,” he added.
The World Health Organization in November issued a conditional recommendation against the use of remdesivir in hospitalised patients, saying there was no evidence that the drug improved survival and other outcomes. Still, many countries, including India, have continued its use.
Dr Reddy’s Laboratories is producing the generic drug Remdesivir under the brand name Redyx in India. Remdesivir used to treat patients suffering from severe symptoms of COVID-19. Redyx will be available in the market for ₹5,400 per 100 mg vials.
In August 2020, Zydus Cadila launched its own version of Remdesivir called Remda in the Indian market.
Dr Reddy’s Laboratories, Biocon firm Syngene and has also received voluntary permission from Gilead Sciences to produce Remdesivir in India.