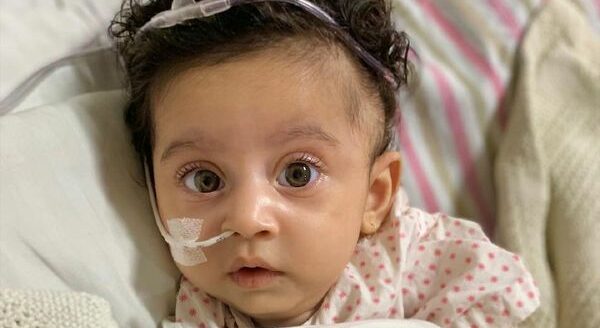
Showing a big heart, Modi Government has decided to waive Rs 6 crore as a GST against Rs 16 crore worth of medical drugs imported for the treatment of a five-month-old Teera Kamat, suffering from a rare genetic disorder.
This entire Rs 16 crore sum was generated by the parent of Teera Kamat in Mumbai through the crowd-funding for her operations. She can be cured through Gene substitute therapy, which includes the medication Zolgensma, imported from the USA.
That cost, as per the Indian currency, is Rs 16 crore. Teera’s parents discussed their daughter’s medical condition with Prime Minister Narendra Modi in October 2020 and again in January 2021.
Devendra Fadnavis thanks PM for waiving off custom duty
Maharashtra’s Leader of Opposition, Devendra Fadnavis wrote to Prime Minister Narendra Modi and Minister of Finance Nirmala Sitaraman to seek the exemption of all relevant taxes on imports from the US of this costly drug. The parent of Teera has raised the Rs 16 crore amount through crowdfunding and has no more money to spend.
Modi responded by saying that Teera will be exempted from the customs duty on imported life-saving drugs.
Fadnavis wrote back, saying, from the bottom of my heart, I thanked the PM for his highly humanitarian and sensitive approach and swift action to exclude customs duties for importing the life-saving medication”.
SMA is presently the leading genetic cause of infant death worldwide
On August 14, 2020, Priyanka and Mihir Kamat, parents of Teera, a resident of Andheri, Mumbai, gave birth to Teera. She was just like every other kid by the time she was born. She used to get restless while drinking milk two weeks after her birth until she stopped breathing.
The doctors advised them she was suffering from Spinal Muscular Astrophys (SMA). A person with this condition does not have genes that are supposed to produce proteins.
This slows down the process of repairing nerves and muscles. As a result, there are limits on swallowing food, breathing, and movement without it, and the condition is getting more severe.
Much research is being conducted abroad on these rare diseases, and several medicines have recently become available in the US.