The COVID-19 pandemic has caused substantial excess mortality and plunged national economies into deep recessions. Although the spread of the virus can be mitigated through physical distancing, face coverings, and testing and tracing—and potentially with therapeutics—the risk of outbreaks and disruption to economic and social life will probably remain until effective vaccines are administered to large portions of the global population to prevent hospitalisation and severe disease, and preferably achieve herd immunity to halt transmission of the virus.
The COVID-19 pandemic is unlikely to end until there is global roll-out of vaccines that protect against severe disease and preferably drive herd immunity. Regulators in numerous countries have authorised or approved COVID-19 vaccines for human use, with more expected to be licensed in 2021.
Yet having licensed vaccines is not enough to achieve global control of COVID-19: they also need to be produced at scale, priced affordably, allocated globally so that they are available where needed, and widely deployed in local communities.
In this Health Policy paper by the Lancet, potential challenges to success in each of these dimensions and discuss policy implications have been discussed. To guide the review, they have developed a dashboard to highlight key characteristics of 26 leading vaccine candidates, including efficacy levels, dosing regimens, storage requirements, prices, production capacities in 2021, and stocks reserved for low-income and middle-income countries.
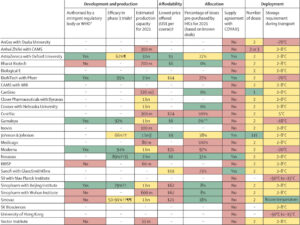
They have used a traffic-light system to signal the potential contributions of each candidate to achieving global vaccine immunity, highlighting important trade-offs that policy makers need to consider when developing and implementing vaccination programmes.
Although specific data points are subject to change as the pandemic response progresses, the dashboard will continue to provide a useful lens to analyse the key issues affecting the use of COVID-19 vaccines.
They have also presented the original data from a 32-country survey (n=26 758) on potential acceptance of COVID-19 vaccines, conducted from October to December, 2020.
Vaccine acceptance was highest in Vietnam (98%), India (91%), China (91%), Denmark (87%), and South Korea (87%), and lowest in Serbia (38%), Croatia (41%), France (44%), Lebanon (44%), and Paraguay (51%).
Several COVID-19 vaccines have now been authorised or approved for human use, with many more in the late stages of clinical development.
Yet having licensed vaccines is not enough to achieve global control of COVID-19: they also need to be produced at scale, priced affordably, allocated globally so that they are available where needed, and widely deployed in local communities.
NEW—#COVID19 vaccination potential will not be achieved without increased production, affordable pricing, global availability, & successful rollout. This Health Policy compares potential of 26 leading vaccines + vaccine confidence in 32 countries. Read https://t.co/QFeRDHutAi pic.twitter.com/rgknOgCVkg
— The Lancet (@TheLancet) February 12, 2021
These four dimensions of the global vaccination challenge are closely related, and the development and production steps have important implications for pricing, allocation, and public confidence.
Development and production
Several manufacturers have successfully developed COVID-19 vaccines in less than 12 months—an extraordinary achievement, given it typically takes a decade or longer to develop new vaccines.5, 6, 7, 8 The world now needs more doses of COVID-19 vaccines than it has done for any other vaccine in history to inoculate enough people for global vaccine immunity.
Affordability
Mechanisms are needed to ensure the affordability and sustainable financing of COVID-19 vaccines in low-income and middle-income countries, which are home to about 85% of the global population and which might lack the resources to buy adequate quantities of vaccines.18, 19 Even in high-income countries, it is important to ensure access to COVID-19 vaccines for poor and marginalised populations.
Global allocation
In addition to the development and affordability of vaccines, an essential pillar of the vaccination challenge is ensuring that enough doses are available globally. Current decisions regarding allocation are being made in the context of constrained supply, with demand exceeding current and projected levels of output.16, 32 Scarcity in supply coupled with the large volumes of pre-orders made by richer countries creates challenges to achieving timely, universal access. Billions of individuals around the world might not have access to COVID-19 vaccines in 2021, which could prolong the pandemic and raise the risk of further mutations of the virus emerging, possibly undermining the efficacy of existing vaccines.
Deployment
Beyond issues related to determining which countries will get vaccine doses when and at what prices, it is essential to ensure the smooth deployment of COVID-19 vaccines. The rapid pace of production and development has shortened the time available for national, regional, and local health officials to plan training and preparedness for COVID-19 vaccination programmes.