The World Health Organization (WHO) has declared mpox a Public Health Emergency of International Concern (PHEIC) for the second time. This decision comes as cases of mpox, previously known as monkeypox, have risen sharply worldwide. Originally identified in 1970 in the Democratic Republic of the Congo, mpox has since spread to 116 countries, including India, and has become a significant global health threat.
What is Mpox?
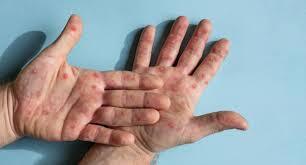
Mpox is a virus that belongs to the Orthopoxvirus genus of the Poxviridae family, the same family that includes viruses causing smallpox, cowpox, and vaccinia. The virus was originally known as monkeypox. But the WHO renamed it to mpox to avoid any stigma associated with its previous name.
The Spread of Mpox Across the Globe
Mpox was first detected in humans in the Democratic Republic of the Congo over five decades ago. The virus initially spread to neighboring African countries but eventually broke through continental barriers, reaching Europe, America, and Asia. In 2022, India reported its first case of it, further emphasizing the virus’s ability to spread rapidly across different regions.
Is There a Vaccine for Mpox?
Yes, there are vaccines available for this disease. The World Health Organization recommends two main vaccines: JYNNEOS and ACAM2000, both approved by health authorities in various countries.
JYNNEOS (MVA-BN Vaccine):
JYNNEOS, also known by other names like Imvamune and Imvanex. Is a vaccine manufacture by Bavarian Nordic. It has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). JYNNEOS preferres because it causes fewer serious side effects compared to other vaccines.
ACAM2000:
ACAM2000 is another vaccine approves by the FDA.
Originally develops to combat smallpox, it is now also uses for mpox vaccination.
However, it is known to cause more serious adverse effects than JYNNEOS.
Other Approved Treatments
In January 2022, the European Medicines Agency (EMA) approved an antiviral treatment called tecovirimat for this disease. This antiviral was originally developed for smallpox but is now use under special circumstances to treat it.
The LC-16 vaccine, another option, has been approved by the WHO and is produced by KM Biologics in Japan. This vaccine is a weakened version of the vaccinia virus. Used as a third-generation smallpox vaccine.
In November 2022, the Russian Federation licensed the OrthopoxVac vaccine for immunization against smallpox, mpox, and other orthopoxviruses.
WHO’s Role in Vaccine Distribution
The WHO Director-General has initiated the process for emergency approval of these vaccines, making it easier for low-income countries to access them. This process allows organizations like Gavi and UNICEF to purchase and distribute these vaccines even if individual countries haven’t granted their own national approval.
Transmission:
Mpox is a zoonotic disease, meaning it can be transmits from animals to humans. Human-to-human transmission occurs through direct contact with the skin, rashes, or scabs of an infected person. According to the US Centers for Disease Control and Prevention (CDC), the virus can also spread through contact with saliva, respiratory secretions like mucus, and bodily fluids. Pregnant women with it can pass the virus to their unborn child during pregnancy or to the newborn during and after birth.
Read Also – WHO Declares Mpox A Global Health Concern: Is India At Risk of Another Pandemic Outbreak?
With the recent resurgence of mpox cases, the WHO’s declaration of a public health emergency serves as a crucial reminder of the need for global vigilance. Vaccines and treatments are available, and international efforts are underway to ensure that these are accessible to all regions, especially in lower-income countries.