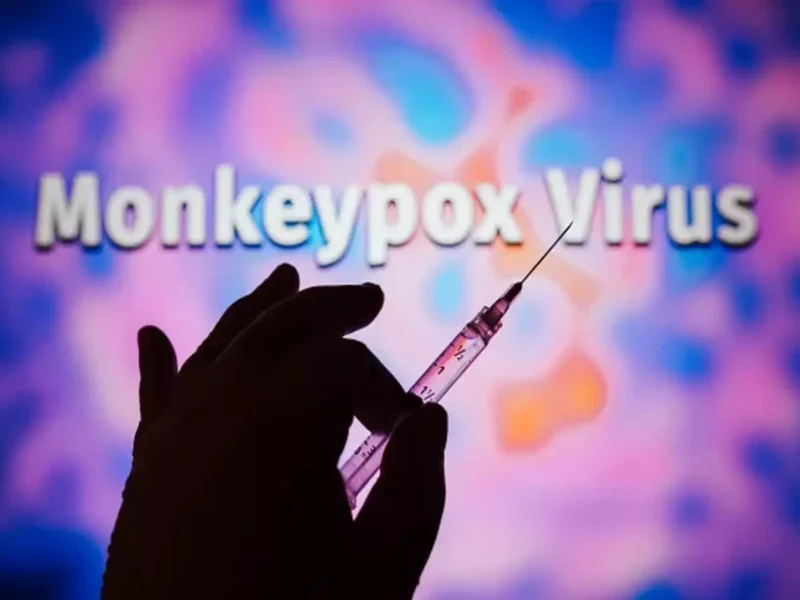
New Delhi, September 14: In a significant milestone in the fight against Mpox, the World Health Organization (WHO) has prequalified the MVA-BN – Mpox Vaccine for use in public health emergencies. This marks a crucial step in ensuring equitable access to the vaccine globally and bolstering efforts to contain the spread of mpox.
The MVA-BN Vaccine: What You Need To Know
The MVA-BN vaccine, developed by Bavarian Nordic, is a modified vaccinia Ankara (MVA) vaccine that has been shown to be effective in preventing mpox in both healthy adults and immunocompromised individuals. It is administered as a single dose and is well-tolerated, with minimal side effects.
Mpox Vaccine: WHO Prequalification
WHO prequalification is a rigorous process that ensures the quality, safety, and efficacy of medical products. By prequalifying the MVA-BN vaccine, WHO has determined that it meets the highest international standards and is suitable for use in public health emergencies.
Global Access and Equity
The prequalification of the Mpox vaccine is a major step towards ensuring equitable access to the vaccine, particularly in low- and middle-income countries. By prequalifying the vaccine, WHO can facilitate its procurement and distribution through its procurement mechanisms, making it more affordable and accessible to countries in need.
The Mpox Outbreak
The global mpox outbreak, which began in May 2022, has raised concerns about the potential for a wider spread of the virus. While the outbreak has been primarily concentrated in certain regions, there is a risk of it spreading to other parts of the world.
The Importance of Vaccination
Vaccination is a crucial tool in combating the spread of infectious diseases. The Mpox vaccine offers a promising means of preventing the virus and mitigating its impact on public health. By ensuring equitable access to the vaccine, WHO is helping to protect vulnerable populations and prevent the virus from becoming a more widespread public health threat.
Mpox Vaccine: Challenges and Future Directions
Despite the prequalification of the Mpox vaccine, several challenges remain. One of the key challenges is ensuring that the vaccine is delivered to those who need it most, particularly in remote or marginalized communities. Additionally, there is a need for continued research and development to address any emerging variants of the virus and to develop more effective vaccines and treatments.
The prequalification of the Mpox vaccine is a significant achievement in the fight against this emerging infectious disease. By ensuring equitable access to the vaccine and supporting ongoing research and development, WHO is helping to protect public health and prevent the spread of mpox.